It
is not scientifically right although investigating with mean temperature is
common.
In
2 ℃ air, if wet-bulb
temperature is 0 ℃, snowflakes do not
melt.
This
is an observation fact.
It
is not appropriate that the following value discusses warming.
Average value of the temperature
of many different places.
I
think that we should use the energy density of air.
It is rare to treat energy
density in meteorology.
1.Energy which melts 1 g of ice
It
is review of fundamental physics here.
It
is said that the heat of fusion of water is 335 (kJ/kg).
335kJ of energy is necessary to
ice into water of 0 ℃ 1 kg.
I
think our A and B boxes of 1 cubic meter volume, and two ice 1g of 0 ℃.
Air
temperature Ta 1mol contains the A.
Air
temperature Tb contains 0.5mol The B.
I
put ice in the box.
The
air and the water I became 0 ℃ after a while, all
the ice melts into water.
I'll try to calculate the value
Ta, of Tb.
Main
component of the air is nitrogen and oxygen.
Degrees of freedom of nitrogen
and oxygen are 5.
Specific heat at constant volume
of 1 mole of air is Cv = (5/2) R=.2.5 ×8.3143 ≒ 20.8 (J / mol)
Energy
that flows into the ice from the air is as follows: A.
Cv
× (Ta-0) = 20.8 × Ta (J)
Energy
that flows into the ice from the air of B is as follows.
0.5 × Cv × Tb-0) 8 = 10.4 × Tb
(J)
The
energy which melts 1 g of ice is 335J.
Ta
and Tb are as follows.
20.8xTa
(J) =335 (J)
10.4xTb
(J) =335 (J)
Ta≒16.1 (℃) =289.3 (K)
Tb≒32.2 (℃) =304.4 (K)
Although A becomes higher 16 ℃ than B, the quantity of the melting ice is the same.
Thus, the average value of the
temperature of A and B does not have a scientific meaning.
2. The 0℃
atmosphere and the 20℃ atmosphere
Let’s
compare the atmosphere of the next two.
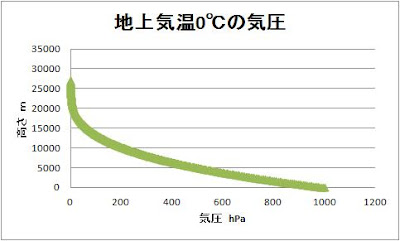
The
atmosphere whose ground pressure and temperature is 1000 hPa at 0 ℃.
The
atmosphere whose ground pressure and temperature is 1000 hPa at 20 ℃.
.
The
atmospheric pressure of height Z is as follows.
P
(Z) = P0exp-∫ (mg / RT (Z ')) dZ'
Integration
range is from 0 to Z.
0
℃ of atmospheric
pressure is as follows.
20 ℃ of atmospheric pressure is as follows.
It
can hardly distinguish.
The
energy of 1 mol of air which it has is as follows.
mgZ+CpT=Const
mgZ
is potential energy, and CpT is enthalpy.
The
equation of state of 1 mol of ideal gas is as follows.
PV=RT
Energy density is as follows.
(P/RT)mgZ+Cp(P/R)
The
former is potential energy density and the latter is enthalpy density.
Enthalpy density comparison is
the same as comparing the atmospheric pressure P.
The next is the graph
which pulled the pressure of 20 ℃ from the pressure of
0 ℃.
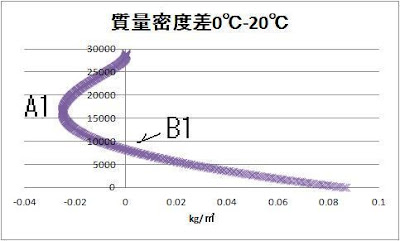
It
can be judged from a graph that the enthalpy density of 20 ℃ is large.
By warming, the height B1 to
which an atmospheric pressure difference becomes large exists.
Pressure
difference that became the largest is 30.7hPa of 8250m.
Let’s compare 0℃ and 20℃ mass density.
A1
was 16500 m, and -0.025kg/㎥.
B1
is 8250 m.
There is height which guesses
warming also about potential energy density.
The energy which carried out
warming is saved up also as potential energy of an air parcel.
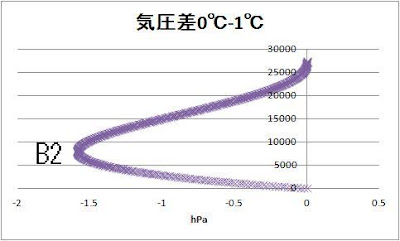
Let’s compare 0℃ and 1℃
The 1.5hPa pressure difference
appeared among 5000 to 10000 m.
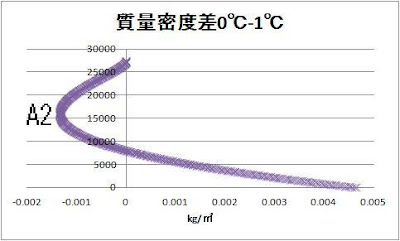
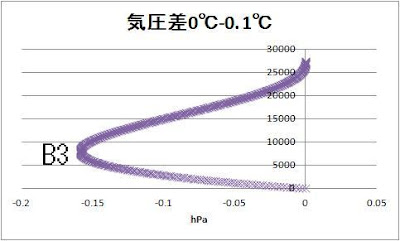
Let’s compare 0℃ and 0.1℃
A1 16500m -0.025kg/㎥
A2 15950m -0.0013 kg/㎥
A3 15950m -0.00013 kg/㎥
B1 8250m -30.7hPa
B2 8000m -1.6hPa
B3 8000m -0.16hPa
0
℃ of water was 0 ℃ of ice, and air also became 0 ℃.
The
temperature before putting in ice is as following.
A
was 16.1 ℃.
B was 32.2 ℃.
********
Nuclear
power generation and Greenhouse effect
Greenhouse
effect has infringed the 2nd law.
And
the principle of conservation of energy is also infringed.
For
example, suppose that a marine temperature went up by warming by 1℃.
Greenhouse
effect cannot explain the immense energy which raises 1℃
of marine temperature.
Scholars ignore such a basic law
and assert Greenhouse effect for nuclear power plant promotion.
They
have not calculated the energy density of air.
The
definition of their potential temperature is wrong.
Even
the scientific proof of warming is not acquired.
They
did not inquire, either but have only said "Propel nuclear power plants."
Where
on earth did their curiosity go?
And
we seem to be the selfish children who think as follows.
Warming is not carried out even
if it consumes energy by Nuclear power generation.
The
mystery in which greenhouse effect is allowed
Let's
check that it is fundamental about temperature.
The
temperature of air is the average of the kinetic energy of air particles.
Air
particles have potential energy and kinetic energy.
The
next can be said from a principle of conservation of energy.
Kinetic energy will become small
if potential energy becomes large.
If
it goes high up in the sky, temperature will fall.
The
temperature of air is dependent on gravity.
The
air of the earth also has the latent heat by vapor.
This complicates structure of the
atmosphere of the earth.
On
the other hand, Venus does not have most water.
It
can approximate by kinetic energy and potential energy.
If
it approaches 100 m on the surface of Venus, it is a rate which 1℃
temperature goes up.
Those
with about 50 km and 500℃ difference in
temperature are required between clouds and the surface.
One hot in the surface of Venus
is for this.
Now,
the basis of greenhouse effect is as follows.
The
thing whose temperature of surface of the earth is higher than radiation
equilibrium temperature is for greenhouse effect.
However,
there is no basis which radiation equilibrium temperature makes the temperature
of earth surface.
Please
consider the surface of a gas planet to be somewhere.
We believe such unscientific
explanation.
The
surface is about 6500 km from the center of the earth.
Radiation
equilibrium temperature is not related to the distance from the center of the
earth.
It
will be set to 0 if radiation equilibrium temperature is differentiated from
the distance R from the center.
Radiation
equilibrium temperature is a value fixed regardless of R.
The
high school student who studied differentiation and physics can understand
this.
Radiation
equilibrium temperature has disregarded the influence of gravity.
Although it is not believed, this
is a fundamental physics level of meteorology.
Greenhouse
effect is a trick for promoting a nuclear power plant.
Is
it necessary why to propel a nuclear power plant?
It
is for processing plutonium and uranium of nuclear weapons curtailed tens of
thousands of shots.
It
is made to process in the nuclear power plant in Asia.
The
name of the military operation is nuclear peaceful use.
Therefore, Greenhouse effect is
required.
A
method passes a research cost to the scholar who studies Greenhouse effect, and
gives scientific authority to Greenhouse effect.
Scholars get social standing and
pretend that there is a scientific basis.
Or since a research cost can be
got, it believes firmly.
A
nuclear power plant group of promoters uses greenhouse effect.
The
United States is not building the nuclear power plant for 30 years or more.
It
is because it turns out that it fails.
It
is clear that they have technical capabilities.
However,
it is Japan and South Korea that make a nuclear power plant to Asia.
When a breakdown becomes actual,
it becomes Japan and South Korea to have promoted atomic power.
It seems that we cannot escape
the influence of the military operation of the name called nuclear peaceful
use.