I think
that wikipedia of Japan has said as follows about fog.
The water drops which is floating in the atmosphere makes
visibility small.
In a weather
survey, a name changes by visibility.
It is called “kiri”
when visibility is less than 1 km.
It is called “moya” when visibility is 1 km or more less than 10
km.
In Japan, fog is called "kiri" or "moya"or"kasumi".
I think as follows.
Water drops have floated into
the air whose visibility is 10 km.
Meteorology of Japan is taught as follows.
A cloud droplet is not made unless it is 100% or more
of humidity.
It is a clear double standard.
It is because the air
whose visibility is 10 km cannot say in 100% of relative humidity by all cases.
The astronaut of your country confirmed the next.
When sprinkling the water of the liquid in space, it
became ice and drifted.
I think that a part evaporates, latent heat is taken
and it freezes.
I considered the following thing.
Can the water of a liquid and gas live together in
weightlessness?
The result became quite strange.
It is a conclusion
infringing the 0th law of thermodynamics.
Thermodynamic equilibrium
http://en.wikipedia.org/wiki/Thermodynamic_equilibrium
1. The liquid and gas in a
gravitational field.
Let's build the vacuous room.
A heat source and a container are in the room.
In the container, the water of a liquid and gas is
contained.
There is a piston in a container and it can change a
size.
The water of a liquid
and gas presupposes that it is in a thermodynamic equilibrium.
1)
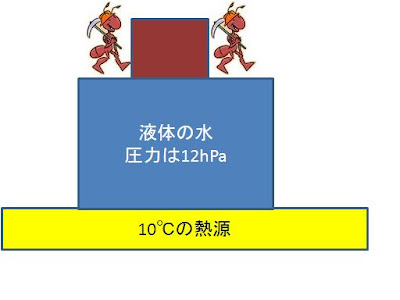
Case 1 .
An
ant and weight are on a container.
Area
of a piston is set to S and the sum total of mass is set to M.
The
pressure in a container serves as P=Mg/S.
A liquid and gas
presuppose that it is in a a thermodynamic
equilibrium.
The
temperature of the water of a liquid and gas should be 10 ℃.
At
this time, the pressure of gas serves as maximum vapor tension.
Here, maximum vapor
tension shall be P= 12 hPa.
2)Case
2
A 10 ℃ heat source is
attached to a container.
An ant leaves from a container.
Pressure becomes small slightly than saturation water
vapor pressure.
A liquid gets energy from a heat source and begins
evaporation.
Let's make all liquid into gas.
Again, an ant gets on a container, and a heat source
is removed.
The inside of a container is set to 12 hPa at 10 ℃.
This state is also a
thermodynamic equilibrium.
3)Case
3
A 10 ℃ heat source is
attached again and two ants get on a container.
Shortly, the pressure P becomes somewhat large and
liquefaction of vapor starts.
The latent heat is emitted to a heat source.
Let's make all gas into liquid.
One ant leaves from a container, and a heat source is
removed.
The inside of a container is set to 12 hPa at 10 ℃.
This state is also a
thermodynamic equilibrium.
Three equilibrium situations have been seen.
All three temperatures and pressures are the same.
You know three differences in each.
Three cases have suggested the next.
The rate of a liquid
and gas is decided by internal energy.
2. The liquid and gas in the weightless.
The experimental device
of the case 1 is brought to the universe.
An ant and weight are
unnecessary.
I think follow.
A liquid becomes a ball.
Of course, a liquid is a premise which does not adhere to a wall.
Now, does the water of
the liquid in this container evaporate?
Although energy is
required to evaporate, there is no supply of energy.
Meteorology was taught as
follows.
Let's consider the ball
of water.
The ball of water will
evaporate under the influence of surface tension.
Moreover, meteorology explains that raindrops resemble strong
sulfuric acid.
Meteorology has the
following fault.
Energy will be given to a
liquid without any restriction.
Meteorology explains as
follows the reason which cannot be made a cloud droplet.
Energy was added to the
liquid and all became gas.
This is a reason which cannot be made a cloud droplet.
I protest as follows.
Meteorology is not premised on thermodynamic equilibrium.
What has the structure of the ball of water become? .
Though regrettable, I do not have the ability to answer.
However, we know the Dry Adiabat Lapse Rate.
The Dry Adiabat Lapse Rate is drawn from the thermodynamic
equilibrium.
Temperature may differ on
the thermodynamic equilibrium.
Now, water vapor pressure
shall be 12 hPa and temperature shall be 10 ℃.
I expect as follows.
The temperature of the
water ball is lower than 10 ℃.
And the following
specific entropy is the same.
The near place of water
ball, and a place distant from water ball.
The small inclination of
temperature and pressure will be made around the water ball.
You add small energy to a
liquid, and may understand.
In order to understand, the energy of surface tension is also required.
On the earth, it becomes
still more complicated.
You have to consider the
mixed gas of vapor and air.
I expect as follows.
The following Value of
specific entropy is the same.
The near place of
wet-bulb's sensor, and a place distant from wet-bulb's sensor.
I think that wet-bulb
temperature is near the thermodynamic equilibrium.
However, it is only imagination.
It has become so difficult that it is unmanageable in me.
Here, my opinion is introduced about ORB.
Please see the following photograph.ORB is reflected.
https://picasaweb.google.com/112066803553804188823/VNdgAF
There is a page which introduced beautiful ORB to that of your country USA.
Orb
Calling
http://www.shot-net.com/ParanormalTrax/orbcalling.htm
My photograph carries out flash photography at the place where the altitude of 1500to2000m and the waterside.
ORB cannot be seen directly.
I consider that orb is water.
And I thought as follows.
If ORB is water, the temperature
is wet-bulb temperature.
The Mt. Tsukuba
weather and a hydrological measurement project http://mtsukuba.suiri.tsukuba.ac.jp/index1.html
Have historical data on the next page.
http://mtsukuba.suiri.tsukuba.ac.jp/sub6.html
Please look for the next page, if you with Excel want to calculate.
Please look for the next page, if you with Excel want to calculate.
The Mt. Tsukuba weather and a hydrological measurement project are observing the following elements.
Temperature of the surfaces, such as temperature, atmospheric pressure, relative humidity, a wind direction, wind velocity, and sunshine, temperature 1cm below ground.
We can calculate wet-bulb temperature 1.5-meter-above ground.
I selected the following data and calculated wet-bulb temperature.
The data of 0.3 m/sec or less of
wind velocity was extracted from the data at 0:00 to 6:00.
The red plot fulfills the following conditions.
-1℃< The difference between a wet bulb and surface temperature <1 ℃
The number of data is the next.
The total number of data is 866.
The number of red plots is 515 (59.5%).
It is 186 (21.5%) that whose temperature of surface was higher than wet-bulb temperature 1 ℃ or more.
It is 165 (19.0%) that whose temperature of surface was lower than wet-bulb temperature 1 ℃ or more.
The correlation coefficient of all the data is 0.98.
The wet-bulb temperature is set to X and Temperature of surface is set to Y.
The regression equation was as follows.
Y=0.9X+0.61Y=0.9X+0.61
Openly, if relative humidity has fallen, wet-bulb temperature will become low.
Cold ORB makes temperature low.
There is no radiative cooling so that the 2nd law may say.
A conclusion does not remain in this.
The surface temperature began to fall from daytime and turned into wet-bulb temperature in the evening.
It reports in the near future.





0 件のコメント:
コメントを投稿